
We need to calculate the change in entropy of both system and surroundings and then add them to get the change in entropy of the Universe.įirst of all we know that this process is completely irreversible therefore $dS \neq \frac \neq 0$, even though the process has been reversible and therefore the Universe must undergo no change in entropy. The lake is a heat bath and the water added is our small thermodynamic system. This is a homework question but I believe it touches on something very general. From: Advances in Enzyme Technology, 2019. What is the change in entropy of the Universe? Entropy changes (S) are estimated through relation GHTS for finite variations at constant T. Question 6: What is the entropy change for the conversion of one gram of ice to water at 237K and one atmospheric pressure (Hfusion 6.435KJ/mol) Solution: H fusion 6. Therefore, the entropy change is 33.7j/K.
Example: Calculate the entropy of a reaction if the amount of heat transfer is 9200 joules and the temperature change is 274K.

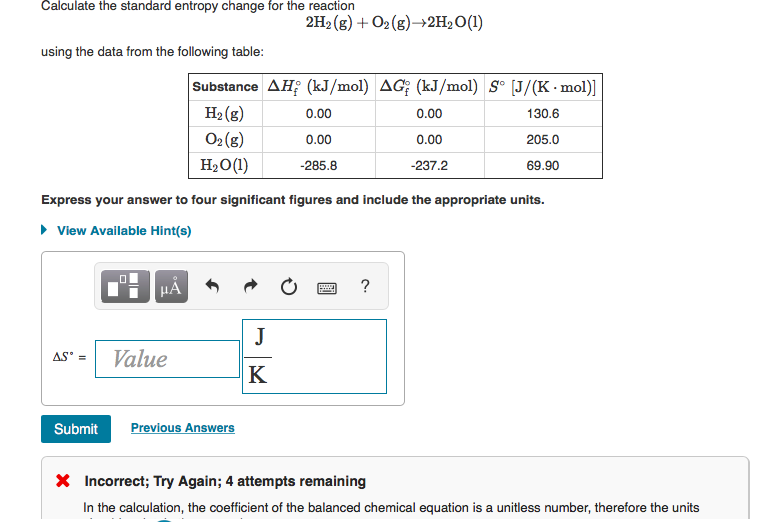
After the reaction, the two are bonded together and can't float around freely from one another.Water at temperature $T_s$ is added to a lake at temperature $T_0 > T_s$ until the total system reaches equilibrium. The following formula calculates the change in entropy: S (q/T)rev (H/T)rev S 15000/343 S 4.37JK -1 mol -1. Entropy is measured in Joules per Kelvin. In other words the N 2( g) used to float around independently of the H 2 gas molecules. This is expected because we are decreasing the number of gas molecules. It would appear that the process results in a decrease in entropy - i.e. \įrom the balanced equation we can write the equation for ΔS 0 (the change in the standard molar entropy for the reaction): As with other calculations related to balanced equations, the coefficients of each component must be taken into account in the entropy calculation (the n, and m, terms below are there to indicate that the coefficients must be accounted for): The entropy change in a chemical reaction is given by the sum of the entropies of the products minus the sum of the entropies of the reactants.

all the ice has melted or all the liquid has frozen)

However, in both of the above situations, the energy change is not accompanied by a change in temperature (the temperature will not change until we no longer have an equilibrium condition i.e. So if, say, you have an enthalpy change of -92.2 kJ mol-1, the value you must put into the equation is -92200 J mol-1. Can you calculate entropy from enthalpy That means that if you are calculating entropy change, you must multiply the enthalpy change value by 1000.


 0 kommentar(er)
0 kommentar(er)
